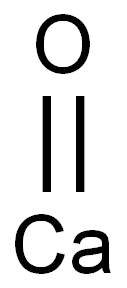Calcium Oxide CAS#1305-78-8
Calcium Oxide CAS#1305-78-8
Promotion Season Now in Store and Free Sample for Testing with Factory Price
Chemical Name:Calcium Oxide
CAS No.:1305-78-8
Molecular Formula:CaO
Sample: Available
Mode of Transportation
1. By Air, fast but expensive.
2. By Sea, usual and economy.
3. By Train, suit for middle Asia countries.
4. By Express, suit for small package.
We only provide highest quality goods available, accompanied by after support!
Calcium Oxide CAS#1305-78-8
Calcium oxide (CaO, CAS Reg. No. 1305-78-8) is also known as lime, quick lime, burnt lime, or calx. Lime does not occur naturally since it reacts so readily with water (to form hydrated lime) and carbon dioxide (to form limestone). It is produced from calcium carbonate, limestone, or oyster shells by calcination at temperatures of 1,700-2,450℃.
Calcium oxide Chemical Properties |
Melting point | 2570 °C |
Boiling point | 2850 °C (lit.) |
density | 3.3 g/mL at 25 °C (lit.) |
bulk density | 800-1200kg/m3 |
refractive index | 1.83 |
Fp | 2850°C |
storage temp. | no restrictions. |
solubility | 1.65g/l Risk of violent reaction. |
form | powder |
color | White to yellow-very slightly beige |
Specific Gravity | 3.3 |
PH | 12.6 (H2O, 20℃)(saturated solution) |
Odor | wh. or gray cryst. or powd., odorless |
Water Solubility | REACTS |
Sensitive | Air & Moisture Sensitive |
Crystal Structure | Cubic |
crystal system | Cube |
Merck | 14,1686 |
Space group | Fm3m |
Lattice constant | a/nmb/nmc/nmα/oβ/oγ/oV/nm30.47760.47760.47769090900.1089 |
Dielectric constant | 2.2(Ambient) |
Exposure limits | ACGIH: TWA 2 mg/m3 |
Stability: | Stability Stable, but absorbs carbon dioxide from the air. Incompatible with water, moisture, fluorine, strong acids. |
InChIKey | ODINCKMPIJJUCX-UHFFFAOYSA-N |
CAS DataBase Reference | 1305-78-8(CAS DataBase Reference) |
NIST Chemistry Reference | Calcium monoxide(1305-78-8) |
EPA Substance Registry System | Calcium oxide (1305-78-8 |
Product Usage
The major uses of lime are metallurgy, flue gas desulfurization, construction, mining, papermaking, and water treatment. Calcium oxide is used to remove impurities during the refining of iron ore. Calcium oxide combines with compounds such as silicates, phosphates, and sulfates contained in iron ores to form slag.
Factory and Equipment Show
Fast transport time
Inventory 2-3 working days New manufacturing 7-10 working days