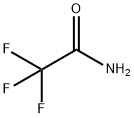
Trifluoroacetamide CAS#354-38-1
Trifluoroacetamide CAS#354-38-1
Promotion Season Now in Store and Free Sample for Testing with Factory Price
Chemical Name:Trifluoroacetamide
CAS No.:354-38-1
Molecular Formula:C2H2F3NO
Sample: Available
Mode of Transportation
1. By Air, fast but expensive.
2. By Sea, usual and economy.
3. By Train, suit for middle Asia countries.
4. By Express, suit for small package.
We only provide highest quality goods available, accompanied by after support!
Trifluoroacetamide CAS#354-38-1
Trifluoroacetamide has a melting point of approximately 72-75℃ and a boiling point of about 162-164℃. It is readily soluble in water, ethanol, ether and other polar organic solvents. It possesses the typical chemical properties of amide compounds. Meanwhile, due to the presence of trifluoromethyl (-CF₃), it has certain stability and special reactivity. For instance, in organic synthesis, it can be used as an acylation reagent or a protecting group.
Trifluoroacetamide Chemical Properties |
Melting point | 65-70 °C (lit.) |
Boiling point | 162.5 °C (lit.) |
density | 1.4176 (estimate) |
bulk density | 960kg/m3 |
Fp | 162-164°C |
storage temp. | Store below +30°C. |
solubility | 460 g/L (20°C) |
pka | 12.33±0.50(Predicted) |
form | Crystalline Powder or Crystals |
color | White to pale yellow or beige |
PH | 3.6 (460g/l, H2O, 23℃) |
Water Solubility | 460 g/L (20 ºC) |
BRN | 1753625 |
InChIKey | NRKYWOKHZRQRJR-UHFFFAOYSA-N |
LogP | 0.725 at 23℃ and pH4.83 |
Dissociation constant | 0 at 23℃ |
CAS DataBase Reference | 354-38-1(CAS DataBase Reference) |
NIST Chemistry Reference | Acetamide, 2,2,2-trifluoro-(354-38-1) |
EPA Substance Registry System | Acetamide, 2,2,2-trifluoro- (354-38-1) |
Safety Information |
Hazard Codes | Xi |
Risk Statements | 36/37/38 |
Safety Statements | 26-36-37/39 |
RIDADR | 1759 |
WGK Germany | 3 |
Hazard Note | Harmful/Irritant |
TSCA | T |
HazardClass | 8 |
PackingGroup | III |
HS Code | 29241900 |
Product Usage
2,2,2-Trifluoroacetamide used in a convenient alternative to the Gabriel synthesis of primary amines from halides by N-alkylation followed by cleavage of the readily-hydrolyzed trifluoroacetyl group and for improved N-alkylation under phase-transfer conditions allowing successive alkylation with different alkyl groups.
Factory and Equipment Show
Fast transport time
Inventory 2-3 working days New manufacturing 7-10 working days