Benzyl Chloride CAS#100-44-7
Benzyl Chloride CAS#100-44-7
Promotion Season Now in Store and Free Sample for Testing with Factory Price
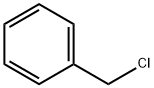
Chemical Name:Benzyl Chloride
CAS No.:100-44-7
Molecular Formula:C7H7Cl
Sample: Available
Mode of Transportation
1. By Air, fast but expensive.
2. By Sea, usual and economy.
3. By Train, suit for middle Asia countries.
4. By Express, suit for small package.
We only provide highest quality goods available, accompanied by after support!
Benzyl Chloride CAS#100-44-7
Benzyl chloride, also known as benzyl chloride, is an organic halogenated hydrocarbon that is flammable, toxic and corrosive. It appears as a colorless to pale yellow transparent liquid with a strong pungent odor. Due to the presence of active benzyl groups in its molecular structure, it has high chemical reactivity. It is insoluble in water but miscible with most organic solvents such as ethanol, ether, benzene and dichloromethane. It can dissolve organic substances such as oils and resins, and is also used as a solvent in a few industrial scenarios.
Benzyl chloride Chemical Properties |
Melting point | -39 °C |
Boiling point | 179 °C |
density | 1.1 g/mL at 25 °C(lit.) |
vapor density | 4.36 (vs air) |
vapor pressure | 10.3 mm Hg ( 60 °C) |
refractive index | n |
Fp | 165 °F |
storage temp. | Store below +30°C. |
solubility | soluble0.46g/L at 30°C (Decomposes in contact with water) |
form | Liquid |
color | Clear colorless to slightly yellow |
Odor | Pungent, irritating. |
explosive limit | 1.1-14%(V) |
Water Solubility | 0.3 g/L (20 ºC) |
Merck | 14,1129 |
BRN | 471308 |
Henry's Law Constant | (x 10-4 atm?m3/mol): 3.57 at 20.00 °C (inert gas stripping, Hovorka and Dohnal, 1997) |
Exposure limits | TLV-TWA 1 ppm (~5mg/m3) (ACGIH, MSHA, and OSHA); IDLH 10 ppm (NIOSH); carcinogenicity: Animal Limited Evidence, Human Inadequate Evidence (IARC). |
Dielectric constant | 7.0(13℃) |
Stability: | Unstable - inhibitors such as propylene oxide or trimethylamine are usually added to prevent polymerization. Combustible. Incompatible with strong oxidizing agents, water, acids, most common metals, dimethyl sulfoxide. Above flash point vapour-air mixtures are explosive within the limits noted above. Contact with water produces toxic fumes. |
InChIKey | KCXMKQUNVWSEMD-UHFFFAOYSA-N |
LogP | 2.3 at 20℃ and pH7 |
Surface tension | 39.36mN/m at 298.15K |
CAS DataBase Reference | 100-44-7(CAS DataBase Reference) |
NIST Chemistry Reference | Benzyl chloride(100-44-7) |
IARC | 2A (Vol. 29, Sup 7, 71) 1999 |
EPA Substance Registry System | Benzyl chloride (100-44-7) |
Safety Information |
Hazard Codes | T,T+ |
Risk Statements | 45-22-23-37/38-41-48/22-43-26-46 |
Safety Statements | 53-45-36/37/39-28-26-36/37 |
OEL | Ceiling: 1 ppm (5 mg/m3) [15-minute] |
RIDADR | UN 1738 6.1/PG 2 |
WGK Germany | 3 |
RTECS | XS8925000 |
F | 8-19 |
Autoignition Temperature | 585 °C |
TSCA | Yes |
HazardClass | 6.1 |
PackingGroup | II |
HS Code | 29039990 |
Hazardous Substances Data | 100-44-7(Hazardous Substances Data) |
Toxicity | LD50 orally in Rabbit: 440 mg/kg |
IDLA | 10 ppm |
Product Usage
Benzyl chloride is mainly used as a pharmaceutical intermediate, raw material for the dye and pigment industry, and raw material for fragrances and daily chemicals. It is also applied in the plastic and rubber industry (for synthesizing PVC stabilizers and rubber vulcanization accelerators), the pesticide industry (for preparing insecticides and herbicides), as well as a small amount of organic synthesis solvents and raw materials for electronic chemicals.
Factory and Equipment Show
Fast transport time
Inventory 2-3 working days New manufacturing 7-10 working days